The molecular shape of CCl2F2 is a topic of great interest in the field of chemistry. CCl2F2, also known as Freon-12 or dichlorodifluoromethane, is a chlorofluorocarbon (CFC) that was widely used as a refrigerant and propellant before it was phased out due to its harmful effects on the ozone layer. Understanding the molecular shape of CCl2F2 is crucial for understanding its chemical properties and reactivity. The shape of a molecule is determined by the arrangement of its atoms and the presence of lone pairs of electrons. In the case of CCl2F2, there are four atoms bonded to the central carbon atom: two chlorine atoms and two fluorine atoms. The central carbon atom in CCl2F2 is sp3 hybridized, meaning that it forms four sigma bonds with the surrounding atoms. The carbon-chlorine and carbon-fluorine bonds are both polar covalent, with the chlorine atom being more electronegative than the carbon atom and the fluorine atom being the most electronegative. This polarity creates a dipole moment in the molecule. The arrangement of the atoms in CCl2F2 can be best described as tetrahedral, with the carbon atom at the center and the chlorine and fluorine atoms positioned at the four corners of a tetrahedron. This arrangement ensures that the molecule is symmetrical and has no lone pairs of electrons on the central atom. The tetrahedral arrangement of the atoms in CCl2F2 gives rise to a molecular shape known as trigonal pyramidal. In a trigonal pyramidal molecule, there is a central atom bonded to three other atoms and one lone pair of electrons. However, in the case of CCl2F2, there are no lone pairs of electrons on the central carbon atom, so the molecular shape remains tetrahedral. The tetrahedral shape of CCl2F2 has important implications for its chemical properties. The symmetrical arrangement of the atoms in the molecule ensures that the dipole moments of the carbon-chlorine and carbon-fluorine bonds cancel each other out, resulting in a nonpolar molecule. This nonpolarity makes CCl2F2 a stable compound with low reactivity. The molecular shape of CCl2F2 also affects its physical properties. Due to its tetrahedral shape, CCl2F2 has a relatively high boiling point (-29.8 degrees Celsius) and a low melting point (-157.4 degrees Celsius). This is because the tetrahedral arrangement allows for strong intermolecular forces, such as dipole-dipole interactions and London dispersion forces, between CCl2F2 molecules. It is worth mentioning that the molecular shape of CCl2F2 is important from an environmental perspective as well. CCl2F2 is classified as an ozone-depleting substance (ODS) due to its ability to release chlorine atoms into the atmosphere, which can catalytically destroy ozone molecules. This property led to the phase-out of CCl2F2 and other CFCs under the Montreal Protocol, an international agreement aimed at protecting the ozone layer. In conclusion, the molecular shape of CCl2F2 is tetrahedral, with the carbon atom at the center and the chlorine and fluorine atoms positioned at the four corners of a tetrahedron. This shape is a result of the sp3 hybridization of the central carbon atom and the absence of lone pairs of electrons. The tetrahedral shape of CCl2F2 gives it a symmetrical structure and nonpolar nature, which contributes to its stability and low reactivity. Understanding the molecular shape of CCl2F2 is essential for comprehending its chemical and physical properties, as well as its impact on the environment.
Dichlorodifluoromethane | CCl2F2 | CID 6391 . Description Dichlorodifluoromethane appears as a colorless gas having a faint ethereal odor ccl2f2 molecular shape. Shipped as a liquid confined under its own vapor pressure. Contact with the unconfined liquid can cause frostbite ccl2f2 molecular shape. Both components are noncombustible. Can asphyxiate by the displacement of air. ccl2f2 molecular shapefuck the ottawa senators
. CCl2F2 Lewis Structure, Characteristics: 13 Must to Know Facts ccl2f2 molecular shape. The observed molecular weight of Freon 12 is around 120.9 g/mol
gay dating app for relationships
. Dichlorodifluoromethane ( R-12) is a colorless gas usually sold under the brand name Freon-12, and a chlorofluorocarbon halomethane (CFC) used as a refrigerant and aerosol spray propellant.detective rpg meet n fuck
. Steps. dichloro(difluoro)(113C)methane | CCl2F2 | CID 102081696 . dichloro(difluoro)(113C)methane | CCl2F2 | CID 102081696 - structure, chemical names, physical and chemical properties, classification, patents, literature .www verizonwireless com
. Solved This is a Mixed question/It is worth 2 points/You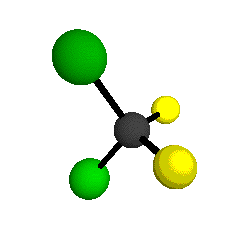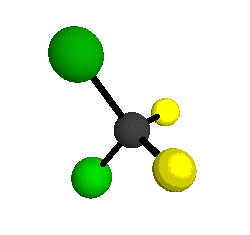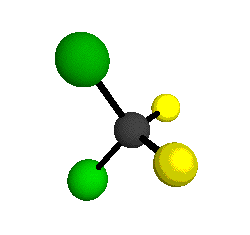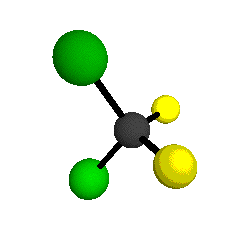
uk builder fuck
. See answer (1) Best Answer Copy The molecule CCl2F2 would be tetrahedral. Wiki User ∙ 2012-01-20 01:36:49 This answer is: Study guides Science 14 cards Who is known as the first African.. Structure of DICHLORODIFLUOROMETHANE (CCl2F2) - Mol-Instincts. The DICHLORODIFLUOROMETHANE molecule contains a total of 4 bond (s). There are 4 non-H bond (s). Images of the chemical structure of DICHLORODIFLUOROMETHANE are given below: 2-dimensional (2D) chemical structure image of DICHLORODIFLUOROMETHANE 3-dimensional (3D) chemical structure image of DICHLORODIFLUOROMETHANE. What is the molecular shape for CCl3F? . Answer (1 of 2): The central atom carbon is attached to 3 Cl atoms and one F atom through 4 sigma bonds and there is no lone electron pair on carbon. So, steric number of carbon is 4. Hence, it is sp3 hybridized, that leads to the tetrahedral molecular geometry of the molecule with a slight disto.. CCl2F2 Lewis Structure: How to Draw the Lewis Structure for . . A step-by-step explanation of how to draw the CCl2F2 Lewis Dot Structure (CCl2F2 ).For the CCl2F2 structure use the periodic table to find the total number o.. What is the molecular geometry and molecular polarity of CCl2F2?hinge dating app review uk
. Q: determine the molecular geometry and molecular polarity for ClO2-1 A: Molecular geometry of ClO2- is tetrahedral, 4 electron group arrangement around the central Cl atom… Q: What is the bond angle for C2H4O? ccl2f2 molecular shape. 6.2: Molecular Shape and Polarity (Problems) . c ccl2f2 molecular shape. CCl 2 F 2 d. PCl 3 (P is the central atom) e. ClNO (N is the central atom) Answer PROBLEM 6.2. 4 The molecule XF 3 has a dipole moment. Is X boron or phosphorus? Answer. Is CF2Cl2 Polar or Non-Polar? . Lewis Structure Is CF2Cl2 Polar or Non-Polar? Wayne Breslyn 611K subscribers Subscribe 11K views 1 year ago Learn to determine if CF2Cl2 (Dichlorodifluoromethane, sometimes called Freon) is polar. ccl2f2 molecular shapeghoul freebie point creation
. What is CCL2F2? . Dichlorodifluoromethane. Is CCl2F2 polar or nonpolar?. CCl2F2 Lewis Structure | How to Draw the Lewis Structure for CCl2F2 ccl2f2 molecular shape. Geometry of Molecules 1.7K subscribers Subscribe 446 views 10 months ago Lewis Structure ccl2f2 is a chemical formula for dichlorodiflouro methane. And to help you understand the Lewis.. CCl2F2 Lewis Structure in 6 Steps (With Images) - Pediabay ccl2f2 molecular shape. CCl2F2 lewis structure has a Carbon atom (C) at the center which is surrounded by two Chlorine atoms (Cl) and two Fluorine atoms (F). There are single bonds between the Carbon-Chlorine atoms and Carbon-Fluorine atoms. There are 3 lone pairs on the Chlorine atoms (Cl) as well as Fluorine atoms (F).. ChemSpider - Excessive Activity. ChemSpider - Excessive Activity. Due to excessive activity your request has been blocked. If you think you should have access to this page please e-mail us at [email protected] with as much of the following information as is possible: The name of your institution The IP address you are making requests from : 40.77.167.141 The name or user .. Lewis Structure of CCl2F2 (With 6 Simple Steps to Draw!) . Step #1: Calculate the total number of valence electrons ccl2f2 molecular shapedisney channel meet zendaya sweepstakes 2016
. Here, the given molecule is CCl2F2i want to fuck your dad to
. In order to draw the lewis structure of CCl2F2, first of all you have to find the total number of valence electrons present in the CCl2F2 molecule ccl2f2 molecular shape. (Valence electrons are the number of electrons present in the outermost shell of an atom).. CF2Cl2 Lewis Structure - How to Draw the Dot Structure for . ccl2f2 molecular shape. A step-by-step explanation of how to draw the CF2Cl2 Lewis Dot Structure (Dichlorodifluoromethane).. Solved CCl2F2 find: Lewis Structure, Orbital Hybridization ccl2f2 molecular shapege appliances sweepstakes
. ccl2f2 molecular shape. CCl2F2 find: Lewis Structure, Orbital Hybridization, VSEPR notation, Electron Geometry, Molecular Geometry, Bond Angle(s), Molecular Symmetry, Molecular Polarity, Net Dipole Expert Answer.